GcMAF (Gc Protein derived Macrophage Activating Factor)
Tests of Second Generation GcMAF
Tests of Second Generation GcMAF
Experiment report
14-JUN-2012 Stability of GcMAF in Serum (PDF)
H Mukai, Y Uto. Department of Biological Science and Technology, The University of Tokushima.
- This experiment was conducted at the University of Tokushima in Japan, using our 2nd generation GcMAF demonstrating macrophage phagocytic activity after short-term and long-term storage conditions. The results show that 2nd generation GcMAF in serum is stable for 1 year at 4 °C, for 14 days at room temperature (around 20 °C), and for 7 days at 40 °C.
- Please note, this research only applies to second generation GcMAF produced with our Patent Pending production process. GcMAF produced using the old method, using vitamin D affinity chromatography, does not have the same stability as 2nd generation GcMAF because it is susceptible to much more rapid oxidation. This difference in stability is due to the different production process, not due to the GcMAF itself.
Macrophage phagocytic activity assay of Second Generation GcMAF
Macrophage phagocytic activity assay using mouse macrophages
Second generation GcMAF is tested for macrophage phagocytic activity using mouse macrophages and sheep red blood cells at the University of Tokushima. The red blood cells are opsonized which marks them for ingestion and destruction by activated macrophages, seen as purple areas in the clear cells. From this we calculate the Phagocytosis (ingestion) Index (PI).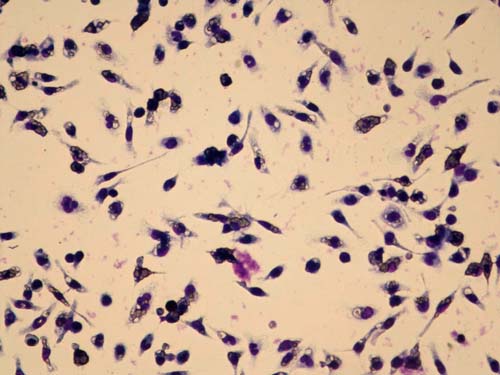
Macrophage phagocytic activity of second generation GcMAF. The purple color are macrophages activated by GcMAF phagocytizing (ingesting) opsonized red blood cells which are clear in color. (Photo courtesy, University of Tokushima)
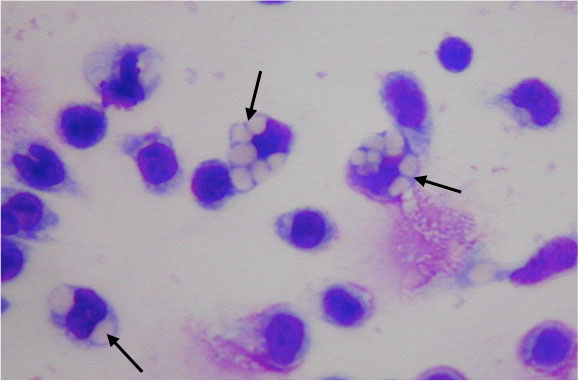
Phagocytosis assay with Second Generation GcMAF. Photo courtesy, University of Tokushima.
Macrophage phagocytic activity assay using macrophage cell line THP-1 and fluorescent beads
Macrophage cell line THP-1 incubated (A) without GcMAF and (B) with Second Generation GcMAF (500 ng/ml GcMAF for 120 min) after addition of fluorescent beads (red), which started to glow red fluorescence after engulfment. Although red fluorescent beads were found within macrophages in both treatment groups A and B (indicated by white arrow), a much larger number of florescent bead were stacked beside the nucleus (blue) in the GcMAF treatment group. This result shows that Second Generation GcMAF strongly activates macrophages and enhances the engulfment of beads used in this assay system. THP-1 cell line macrophages were induced by 10ng/ml 12-O-Tetradecanoylphorbol-13-acetate (TPA).
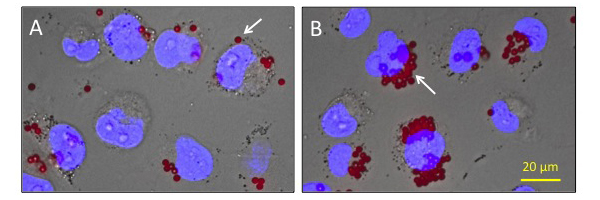
Micropscope picture showing macrophage phagocytic activity assay using macrophage cell line THP-1 and fluorescent beads. (A) Macrophages not activated by GcMAF show little phagocytic activity. (B) Macrophages activated by Second Generation GcMAF show very high phagocytic activity seen by the much larger number of red florescent beads engulfed by the macrophages. Photo courtesy, Frontier Institute for Biomolecular Engineering Research (FIBER), Konan University, Kobe, Japan.
Research paper
For more on this assay system, see A Novel Assay System for Macrophage-activating Factor Activity Using a Human U937 Cell Line
Anticancer Research 34: 4577-4581, 2014.
Increased rate of maturation of Dendritic cells (DCs) in vitro
In vitro experiments conducted by Saisei Mirai indicate increased rate of maturation of dendritic cells (DCs), a type of immune cell, with 2nd Generation GcMAF. This graph shows specific marker expression of dendritic cells grown from normal peripheral blood with and without 2nd Generation GcMAF after 6 and 8 days cultivation. From this preliminary experiment we observed that 2nd Generation GcMAF increases the rate of maturation of dendritic cells. The ability of dendritic cells to regulate immunity is dependent on DC maturation. The process of DC maturation, results in an increase in the surface expression of costimulatory molecules, morphological changes (such as formation of dendrites), secretion of chemokines, cytokines and proteases, and surface expression of adhesion molecules and chemokine receptors.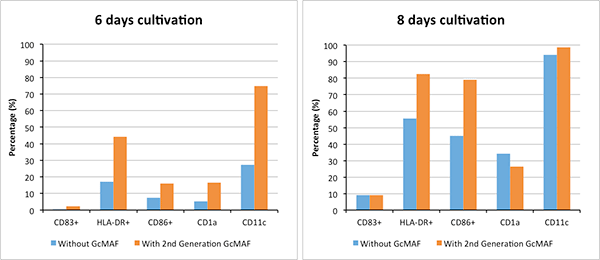
Flow cytometric analysis of dendric cells (DCs) grown from normal peripheral blood with and without 2nd Generation GcMAF on specific marker expression after 6 and 8 days cultivation.
How does this test work?
Mature DCs are detected by the presence or absence of various molecules on their cell surface. These include molecules such as CD83+, HLA-DR+, CD86+, CD1a, CD11c which can be measured.
Helix pomatia agglutinin (HPA) lectin (for GalNAc residue)
Second generation Gc-MAF is identified using electrophoresis Western blot with Helix pomatia agglutinin (HPA) lectin (for GalNAc residue).
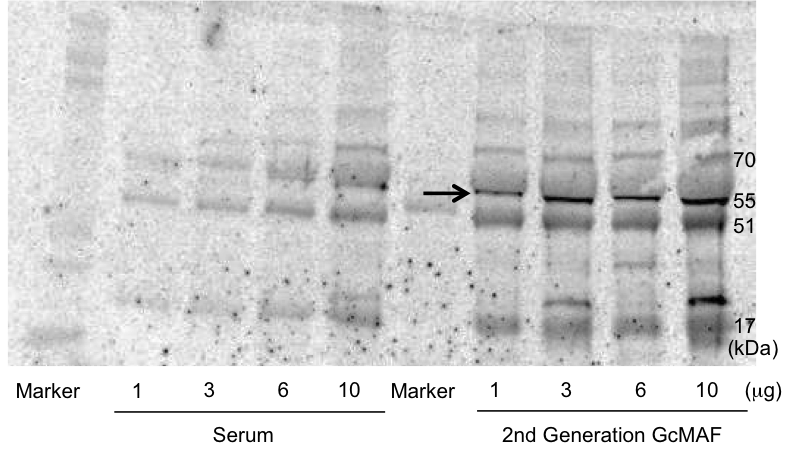
Photo courtesy, University of Tokushima.
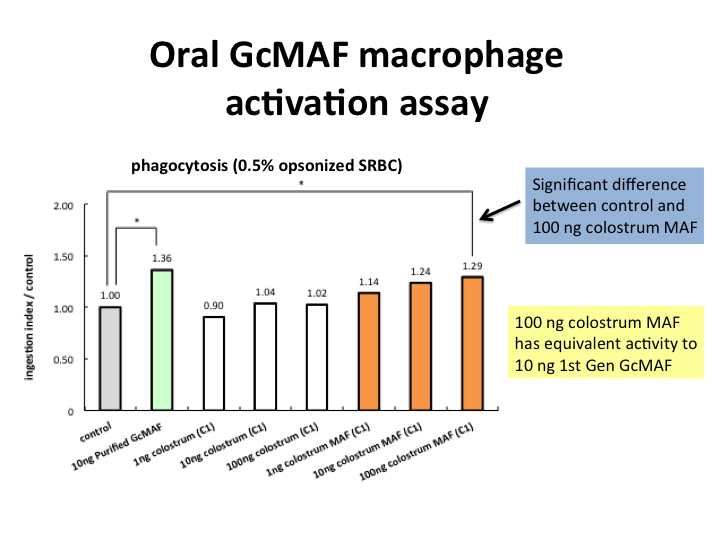
Oral Colostrum GcMAF macrophage activity assay
We find that 100 ng Oral Colostrum MAF has equivalent macrophage phagocytic activity to 10 ng first generation GcMAF. Pure colostrum has no significant activity compared to control, so pure colostrum does not activate macrophages unless modified using our patented technology.
Oral Colostrum MAF (macrophage activating factor) is administered orally in the gut in an Enteric capsules and as a powder in the mouth by opening the capsule. This activates macrophages in the lymphoid tissue of the gut and mouth which are important parts of our immune system.
If you wish to purchase GcMAF therapy or make an enquiry, please contact us with details of your disease, current treatment and the quantities of Gc-MAF you require.
